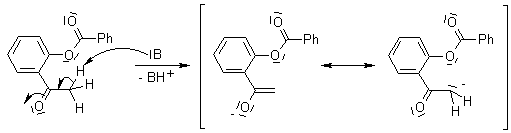
Baker-Venkataraman Rearrangement

The base-induced transfer of the ester acyl group in an o-acylated phenol ester, which leads to a 1,3-diketone. This reaction is related to the Claisen Condensation, and proceeds through the formation of an enolate, followed by intramolecular acyl transfer.
Mechanism of the Baker-Venkataraman Rearrangement

Recent Literature
Directed ortho metalation - cross coupling links. Carbamoyl rendition of the baker-venkataraman rearrangement. Regiospecific route to substituted 4-hydroxycoumarins

No comments:
Post a Comment