Phenols are the organic compounds containing benzene ring bonded to a hydroxyl group. They are also known as carbolic acids. They exhibit unique physical and chemical properties in comparison to alcohol. These physical and chemical properties of phenols are mainly due to the presence of the hydroxyl group.
Some prominent physical and chemical properties of phenols are given below.
1. Boiling Point of Phenols
Phenols generally have higher boiling points in comparison to other hydrocarbons having equal molecular masses. This is due to the presence of intermolecular hydrogen bonding between hydroxyl groups of phenol molecules. In general, the boiling point of phenols increases with an increase in the number of carbon atoms.
2. Solubility of Phenols
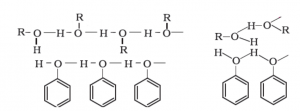
- The solubility of phenol in water is governed by the hydroxyl group present.
- The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding.
- Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water.
- However, the aryl group attached to the hydroxyl group is hydrophobic in nature.
- Thus, the solubility of phenol decreases with the increase in the size of the aryl group.
3. Acidity of Phenols
Phenols react with active metals such as sodium, potassium, etc. to form the corresponding phenoxide. These reactions of phenols indicate its acidic nature. In phenol, the sp2 hybridized carbon of the benzene ring attached directly to the hydroxyl group acts as an electron-withdrawing group. Thus, it decreases the electron density on oxygen.
Due to the delocalization of negative charge in the benzene ring, phenoxide ions are more stable than alkoxide ions. As a result, phenols are more acidic than alcohols. In the case of substituted phenols, the acidity decreases if an electron-donating group is attached to the ring while the acidity increases in the case of an electron-withdrawing group.
4. Chirality of Phenols
Phenols exhibit chirality within their molecules, for example, catechin. This chirality is due to the absence of planar and axial symmetry in the phenol molecule.
No comments:
Post a Comment