Wittig Reaction

The Wittig Reaction allows the preparation of an alkene by the reaction of an aldehyde or ketone with the ylide generated from a phosphonium salt. The geometry of the resulting alkene depends on the reactivity of the ylide. If R is an electron withdrawing group, then the ylide is stabilized and is not as reactive as when R is alkyl. Stabilized ylides give predominantly (E)-alkenes whereas non-stabilized ylides lead to (Z)-alkenes (see also Wittig-Horner Reaction).
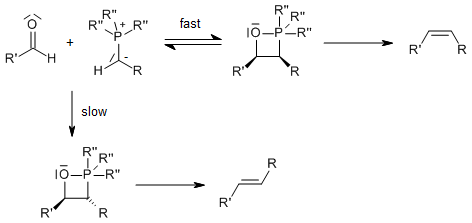
Mechanism of the Wittig Reaction
(2+2) Cycloaddition of the ylide to the carbonyl forms a four-membered cyclic intermediate, an oxaphosphetane. Preliminary posultated mechanisms lead first to a betaine as a zwitterionic intermediate, which would then close to the oxaphosphetane. The intermediacy of such betaines plays an important role in the Schlosser Modification. Betaines may be stabilized by lithium salts leading to side products; therefore, suitable bases in the Wittig Reaction are for example: NaH, NaOMe, NEt3.

The driving force is the formation of a very stable phosphine oxide:

Reactive ylides give rapid reaction and subsequent rapid ring opening to give the (Z)-alkene:
Recent LiteraturE
Recycling the Waste: The Development of a Catalytic Wittig Reaction
PhCH=P(MeNCH2CH2)3N: A Novel Ylide for Quantitative E Selectivity in the Wittig Reaction
Highly Tunable Stereoselective Olefination of Semistabilized Triphenylphosphonium Ylides with N-Sulfonyl Imines
Potassium Hydride in Paraffin: A Useful Base for Organic Synthesis
Generation of Phosphoranes Derived from Phosphites. A New Class of Phosphorus Ylides Leading to High E Selectivity with Semi-stabilizing Groups in Wittig Olefinations
One-Pot Synthesis of α,β-Unsaturated Esters, Ketones, and Nitriles from Alcohols and Phosphonium Salts
A Three-Step Route to a Tricyclic Steroid Precursor
Chromatography-Free Wittig Reactions Using a Bifunctional Polymeric Reagent
Wittig Reactions in Water Media Employing Stabilized Ylides with Aldehydes. Synthesis of α,β-Unsaturated Esters from Mixing Aldehydes, α-Bromoesters, and Ph3P in Aqueous NaHCO3
One-Pot Wittig Reactions in Water and in the Presence of a Surfactant
Direct Conversion of N-Methoxy-N-methylamides (Weinreb Amides) to Ketones via a Nonclassical Wittig Reaction
PPh3O as an Activating Reagent for One-Pot Stereoselective Syntheses of Di- and Polybrominated Esters from Simple Aldehydes
A highly stereoselective tandem Michael addition-Wittig reaction of (3-carboxy-2-oxopropylidene)triphenylphosphorane and α,β-unsaturated aldehydes gives multifunctional 6-carboxycyclohex-2-en-1-ones in excellent diastereo- and enantioselectivities by employing the combined catalysis of a bulky chiral secondary amine, LiClO4, and DABCO.
Wittig Olefination between Phosphine, Aldehyde, and Allylic Carbonate: A General Method for Stereoselective Synthesis of Trisubstituted 1,3-Dienes with Highly Variable Substituents
In the presence of ruthenium-based olefin metathesis catalysts and triphenylphosphine, α,β-unsaturated aldehydes can be olefinated with diazoacetates. A tandem transformation of terminal olefins to 1,3-dienoic olefins in a single operation based on olefin cross-metathesis and Wittig olefination has been developed.
Synthesis of Vinyl Boronates from Aldehydes by a Practical Boron-Wittig Reaction
An Intramolecular Wittig Approach toward Heteroarenes: Synthesis of Pyrazoles, Isoxazoles, and Chromenone-oximes
Synthesis of Functionalized Furans via Chemoselective Reduction/Wittig Reaction Using Catalytic Triethylamine and Phosphine
Synthesis of Polysubstituted Pyridines via a One-Pot Metal-Free Strategy
Synthesis of Polysubstituted Isoquinolines and Related Fused Pyridines from Alkenyl Boronic Esters via a Copper-Catalyzed Azidation/Aza-Wittig Condensation Sequence




















No comments:
Post a Comment