Ring Closing Metathesis (RCM)

The Ring-Closing Metathesis (RCM) allows synthesis of 5- up to 30-membered cyclic alkenes. The E/Z-selectivity depends on the ring strain.
The Ru-catalysts used tolerate a variety of functional groups, but normally the molecule must have polar side chains that are able to build a template for the catalyst. The modern Second Generation Grubb's Catalysts (see Olefin Metathesis) are more versatile.
Mechanism of Ring Closing Metathesis
The key intermediate is a metallacyclobutane, which can undergo cycloreversion either towards products or back to starting materials. When the olefins of the substrate are terminal, the driving force for RCM is the removal of ethene from the reaction mixture.
Initiation:
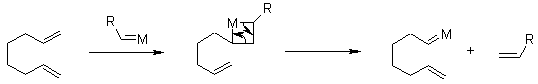
Catalytic cycle:

Chauvin's Mechanism
Recent Literature
Synthesis of 1,2-Disubstituted Cyclopentadienes from Alkynes Using a Catalytic Haloallylation/Cross-Coupling/Metathesis Relay
A Selective and Functional Group-Tolerant Ruthenium-Catalyzed Olefin Metathesis/Transfer Hydrogenation Tandem Sequence Using Formic Acid as Hydrogen Source
Indenylidene Ruthenium Complex Bearing a Sterically Demanding NHC Ligand: An Efficient Catalyst for Olefin Metathesis at Room Temperature
Aminocarbonyl Group Containing Hoveyda-Grubbs-Type Complexes: Synthesis and Activity in Olefin Metathesis Transformations
Visible-Light-Controlled Ruthenium-Catalyzed Olefin Metathesis
Pd, Ru, and Fe catalysis enable a general synthesis of 2-substituted pyrroles in overall good yields with only water and ethene as side-products. The route starts with two subsequent Pd-catalyzed monoallylations of amines with allylic alcohols. Ru-catalyzed ring-closing metathesis performed on the diallylated amines provides pyrrolines in excellent yields. By addition of ferric chloride, a selective aromatization was achieved.
Supported Ruthenium-Carbene Catalyst on Ionic Magnetic Nanoparticles for Olefin Metathesis
Synthesis of Imidazolium-Tagged Ruthenium Carbene Complex: Remarkable Activity and Reusability in Regard to Olefin Metathesis in Ionic Liquids
Allenylidene-to-Indenylidene Rearrangement in Arene-Ruthenium Complexes: A Key Step to Highly Active Catalysts for Olefin Metathesis Reactions
Advanced Fine-Tuning of Grubbs/Hoveyda Olefin Metathesis Catalysts: A Further Step toward an Optimum Balance between Antinomic Properties
PQS: A New Platform for Micellar Catalysis. RCM Reactions in Water, with Catalyst Recycling
Olefin Ring Closing Metathesis and Hydrosilylation Reaction in Aqueous Medium by Grubbs Second Generation Ruthenium Catalyst
Δ3-Aryl/heteroaryl substituted heterocycles via sequential Pd-catalysed termolecular cascade/ring closing metathesis (RCM)
Synthesis of Nitrogen-Containing Heterocycles via Ring-Closing Ene-Ene and Ene-Yne Metathesis Reactions: An Easy Access to 1- and 2-Benzazepine Scaffolds and Five- and Six-Membered Lactams
Synthesis of Sultams by Ring-Closing Metathesis
S. Mondal, S. Debnath, Synthesis, 2014, 46, 368-374.(BENGALIS !)
Studies on the Synthesis of Endocyclic Enol Lactones via a RCM of Selected Vinyl Esters
Synthesis of Fused Bicyclic Imidazoles by Sequential Van Leusen/Ring-Closing Metathesis Reactions
Enantioselective Synthesis of Cyclic Amides and Amines through Mo-Catalyzed Asymmetric Ring-Closing Metathesis
An Efficient Route to Benzene and Phenol Derivatives via Ring-Closing Olefin Metathesis
A New Synthetic Approach to Phenol Derivatives: Use of Ring-Closing Olefin Metathesis
An isomerization-ring-closing metathesis strategy for the synthesis of substituted benzofurans
Synthesis of α,β-Unsaturated 4,5-Disubstituted γ-Lactones via Ring-Closing Metathesis Catalyzed by the First-Generation Grubbs' Catalyst
An Olefin Metathesis/Double Bond Isomerization Sequence Catalyzed by an In Situ Generated Ruthenium Hydride Species
Imino Glycals via Ruthenium-Catalyzed RCM and Isomerization
Multicatalytic Processes Using Diverse Transition Metals for the Synthesis of Alkenes
Highly Active Ruthenium Metathesis Catalysts Exhibiting Unprecedented Activity and Z-Selectivity
Chelate-Assisted Ring-Closing Metathesis: A Strategy for Accelerating Macrocyclization at Ambient Temperatures
Synthesis of Selectively Substituted or Deuterated Indenes via Sequential Pd and Ru Catalysis



























No comments:
Post a Comment