Baeyer-Villiger Oxidation

The Baeyer-Villiger Oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts ketones to esters and cyclic ketones to lactones. The Baeyer-Villiger can be carried out with peracids, such as MCBPA, or with hydrogen peroxide and a Lewis acid.
The regiospecificity of the reaction depends on the relative migratory ability of the substituents attached to the carbonyl. Substituents which are able to stabilize a positive charge migrate more readily, so that the order of preference is: tert. alkyl > cyclohexyl > sec. alkyl > phenyl > prim. alkyl > CH3. In some cases, stereoelectronic or ring strain factors also affect the regiochemical outcome.
Mechanism of the Baeyer-Villiger Oxidation



Recent Literature
Baeyer-Villiger Oxidation of Ketones to Esters with Sodium Percarbonate/Trifluoroacetic Acid
Selenoxides as Catalysts for Epoxidation and Baeyer-Villiger Oxidation with Hydrogen Peroxide
Hypervalent λ3-Bromane Strategy for Baeyer-Villiger Oxidation: Selective Transformation of Primary Aliphatic and Aromatic Aldehydes to Formates, Which is Missing in the Classical Baeyer-Villiger Oxidation
General Metal-Free Baeyer-Villiger-Type Synthesis of Vinyl Acetates
Enantioselective Baeyer-Villiger Oxidation: Desymmetrization of Meso Cyclic Ketones and Kinetic Resolution of Racemic 2-Arylcyclohexanones
Asymmetric Baeyer-Villiger Reaction with Hydrogen Peroxide Catalyzed by a Novel Planar-Chiral Bisflavin
Conversion of Cyclic Acetals to Hydroxy Esters by MCPBA Oxidation


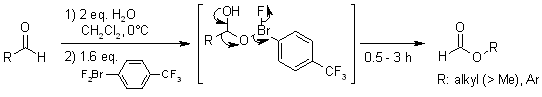




No comments:
Post a Comment