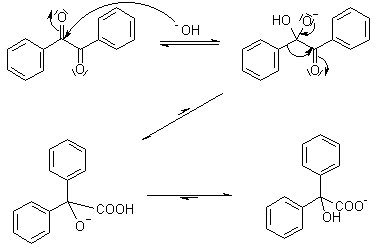
Benzilic Acid Rearrangement

1,2-Diketones undergo a rearrangement in the presence of strong base to yield α-hydroxycarboxylic acids. The best yields are obtained when the subject diketones do not have enolizable protons.
The reaction of a cyclic diketone leads to an interesting ring contraction:

Ketoaldehydes do not react in the same manner, where a hydride shift is preferred (see Cannizzaro Reaction)
Mechanism of Benzilic Acid Rearrangement
Recent Literature
Direct Synthesis of 1,2-Diketones by Catalytic Aerobic Oxidative Decarboxylation of 1,3-Diketones with Iodine and Base under Irradiation of Fluorescent Light

No comments:
Post a Comment