Wolff-Kishner Reduction
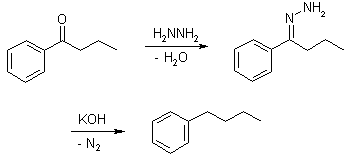
The reduction of aldehydes and ketones to alkanes. Condensation of the carbonyl compound with hydrazine forms the hydrazone, and treatment with base induces the reduction of the carbon coupled with oxidation of the hydrazine to gaseous nitrogen, to yield the corresponding alkane.
The Clemmensen Reduction can effect a similar conversion under strongly acidic conditions, and is useful if the starting material is base-labile.
Mechanism of the Wolff-Kishner Reduction

Recent Literature
Practical Procedures for the Preparation of N-tert-Butyldimethylsilylhydrazones and Their Use in Modified Wolff-Kishner Reductions and in the Synthesis of Vinyl Halides and gem-Dihalides
En Route to a Practical Primary Alcohol Deoxygenation
Methyl Hydrazinocarboxylate as a Practical Alternative to Hydrazine in the Wolff-Kishner Reaction



No comments:
Post a Comment