Diazotisation

The nitrosation of primary aromatic amines with nitrous acid (generated in situ from sodium nitrite and a strong acid, such as hydrochloric acid, sulfuric acid, or HBF4) leads to diazonium salts, which can be isolated if the counterion is non-nucleophilic.
Diazonium salts are important intermediates for the preparation of halides (Sandmeyer Reaction, Schiemann Reaction), and azo compounds. Diazonium salts can react as pseudohalide-type electrophiles, and can therefore be used in specific protocols for the Heck Reaction or Suzuki Coupling.
The intermediates resulting from the diazotization of primary, aliphatic amines are unstable; they are rapidly converted into carbocations after loss of nitrogen, and yield products derived from substitution, elimination or rearrangement processes.
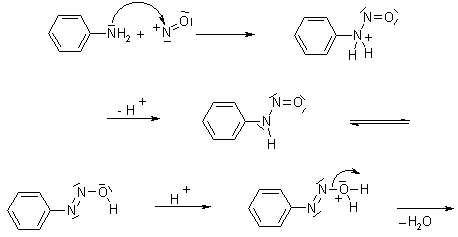
Mechanism of Diazotisation


Recent Literature
A convenient and general one-step preparation of aromatic and some heterocyclic iodides in good yields includes a sequential diazotization-iodination of aromatic amines with KI, NaNO2, and p-TsOH in acetonitrile at room temperature.
Sulfonic Acid Based Cation-Exchange Resin: A Novel Proton Source for One-Pot Diazotization-Iodination of Aromatic Amines in Water
Unusually Stable, Versatile, and Pure Arenediazonium Tosylates: Their Preparation, Structures, and Synthetic Applicability
A Simple and Effective Synthesis of Aryl Azides via Arenediazonium Tosylates
Methanol-Promoted Borylation of Arylamines: A Simple and Green Synthetic Method to Arylboronic Acids and Arylboronates
Efficient Synthesis of 2-Amino Acid by Homologation of β2-Amino Acids Involving the Reformatsky Reaction and Mannich-Type Imminium Electrophile
Synthesis and properties of bis(hetaryl)azo dyes







No comments:
Post a Comment