Brown Hydroboration

The syn-addition of hydroboranes to alkenes occurs with predictable selectivity, wherein the boron adds preferentially to the least hindered carbon. This selectivity is enhanced if sterically demanding boranes are used.

Coupling the hydroboration with a subsequent oxidation of the new formed borane yields anti-Markovnikov alcohols. The hydroboration/oxidation sequence constitutes a powerful method for the regio- and stereoselective synthesis of alcohols.
The product boranes may also be used as starting materials for other reactions, such as Suzuki Couplings (see Recent Literature).
Mechanism of the Brown Hydroboration
The selectivity of the first addition of borane can be relatively low:


The subsequent additions are more selective as the steric bulk increases, and anti-Markovnikov selectivity predominates in the end:

Oxidation with hydrogen peroxide leads to alcohols:

Sterically demanding boranes offer enhanced selectivity. One example of a sterically demanding borane (9-BBN) is generated by the double addition of borane to 1,5-cyclooctadiene:

9-Borabicyclo[3.3.1]nonane


The reactivity and selectivity of the borane reagent may be modified through the use of borane-Lewis base complexes.
Recent Literature
Hydroboration. 97. Synthesis of New Exceptional Chloroborane-Lewis Base Adducts for Hydroboration. Dioxane-Monochloroborane as a Superior Reagent for the Selective Hydroboration of Terminal Alkenes
Hydroboration with Pyridine Borane at Room Temperature
Hydroboration with Pyridine Borane at Room Temperature
Sodium perborate: a mild and convenient reagent for efficiently oxidizing organoboranes
Dod-S-Me and Methyl 6-Morpholinohexyl Sulfide (MMS) as New Odorless Borane Carriers
Synthesis of Tertiary Alkyl Amines from Terminal Alkenes: Copper-Catalyzed Amination of Alkyl Boranes
Highly Selective Bis(imino)pyridine Iron-Catalyzed Alkene Hydroboration
Borane-Catalysed Hydroboration of Alkynes and Alkenes
Preparation of (E)-1-Alkenylboronic Acid Pinacol Esters via Transfer of Alkenyl Group from Boron to Boron
Highly Stereoselective Synthesis of cis-Alkenyl Pinacolboronates and Potassium cis-Alkenyltrifluoroborates via a Hydroboration/Protodeboronation Approach
Earth-Abundant Metal Catalysis Enabled by Counterion Activation
Iron-Catalyzed 1,4-Hydroboration of 1,3-Dienes
Nickel-Catalyzed 1,4-Addition of Trialkylboranes to α,β-Unsaturated Esters: Dramatic Enhancement by Addition of Methanol
Regioselective Semihydrogenation of Dienes
Regioselective and Stereoselective Entry to β,β-Disubstituted Vinyl Ethers via the Sequential Hydroboration/Suzuki-Miyaura Coupling of Ynol Ethers
Concise Formation of 4-Benzyl Piperidines and Related Derivatives Using a Suzuki Protocol
An Exceptional Hydroboration of Substituted Fluoroolefins Providing Tertiary Alcohols


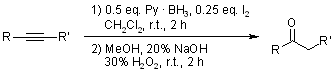














No comments:
Post a Comment