Friedel-Crafts Alkylation

This Lewis acid-catalyzed electrophilic aromatic substitution allows the synthesis of alkylated products via the reaction of arenes with alkyl halides or alkenes. Since alkyl substituents activate the arene substrate, polyalkylation may occur. A valuable, two-step alternative is Friedel-Crafts Acylation followed by a carbonyl reduction.
Mechanism of the Friedel-Crafts Alkylation


Using alkenes :
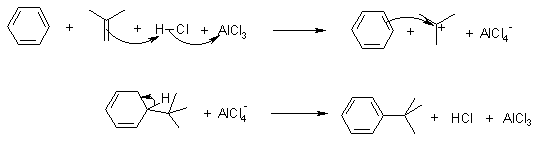
Recent Literature
Iron Oxide Nanoparticles Grown on Carboxy-Functionalized Graphite: An Efficient Reusable Catalyst for Alkylation of Arenes
Dicationic Electrophiles from Olefinic Amines in Superacid
Benzylic Phosphates in Friedel-Crafts Reactions with Activated and Unactivated Arenes: Access to Polyarylated Alkanes
Samarium Triflate-Catalyzed Halogen-Promoted Friedel-Crafts Alkylation with Alkenes
Dramatic Enhancement of Catalytic Activity in an Ionic Liquid: Highly Practical Friedel-Crafts Alkenylation of Arenes with Alkynes Catalyzed by Metal Triflates
MA-Silica Gel Catalyzed Propargylation of Aromatic Compounds with Arylpropargyl Alcohols under Solvent-Free Conditions
Iron-Catalyzed Synthesis of Tetrahydronaphthalenes via 3,4-Dihydro-2H-pyran Intermediates
Metal-Free Tandem Friedel–Crafts/Lactonization Reaction to Benzofuranones Bearing a Quaternary Center at C3 Position
Addition of Arylboronic Acids to Arylpropargyl Alcohols en Route to Indenes and Quinolines
Conversion of 2-Alkylcinnamaldehydes to 2-Alkylindanones via a Catalytic Intramolecular Friedel-Crafts Reaction









No comments:
Post a Comment