Mannich Reaction

This multi-component condensation of a nonenolizable aldehyde, a primary or secondary amine and an enolizable carbonyl compound affords aminomethylated products. The iminium derivative of the aldehyde is the acceptor in the reaction.
The involvement of the Mannich Reaction has been proposed in many biosynthetic pathways, especially for alkaloids.
Mechanism of the Mannich Reaction

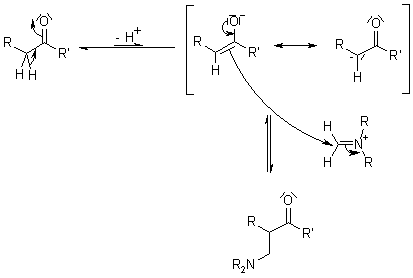
Recent Literature
An Efficient, Mild, Solvent-Free, One-Pot Three-Component Mannich Reaction Catalyzed by (C4H12N2)2[BiCl6]Cl·H2O
Transition Metal Salt-Catalyzed Direct Three-Component Mannich Reactions of Aldehydes, Ketones, and Carbamates: Efficient Synthesis of N-Protected β-Aryl-β-Amino Ketone Compounds
Catalytic Silicon-Mediated Carbon-Carbon Bond-Forming Reactions of Unactivated Amides
Highly Enantioselective Three-Component Direct Mannich Reactions of Unfunctionalized Ketones Catalyzed by Bifunctional Organocatalysts
Ag-Catalyzed Asymmetric Mannich Reactions of Enol Ethers with Aryl, Alkyl, Alkenyl, and Alkynyl Imines
The Direct and Enantioselective, One-Pot, Three-Component, Cross-Mannich Reaction of Aldehydes
Disulfonimide-Catalyzed Asymmetric Synthesis of β3-Amino Esters Directly from N-Boc-Amino Sulfones
Asymmetric Phase-Transfer Catalysts Bearing Multiple Hydrogen-Bonding Donors: Highly Efficient Catalysts for Enantio- and Diastereoselective Nitro-Mannich Reaction of Amidosulfones
Organocatalysis with proline derivatives: improved catalysts for the asymmetric Mannich, nitro-Michael and aldol reactions
Catalysis of 3-Pyrrolidinecarboxylic Acid and Related Pyrrolidine Derivatives in Enantioselective anti-Mannich-Type Reactions: Importance of the 3-Acid Group on Pyrrolidine for Stereocontrol
Diarylborinic Acid Derivatives as a Catalytic Iminium Ion Generator in the Mannich-Type Reaction Using Secondary Amines, Aldehydes, and Ketene Silyl Acetals
Stereoselective Synthesis of β-Amino Ketones via Direct Mannich-Type Reactions, Catalyzed with ZrOCl2 · 8 H2O under Solvent-Free Conditions
Magnesium(II)-Binaphtholate as a Practical Chiral Catalyst for the Enantioselective Direct Mannich-Type Reaction with Malonates
Diastereoselectively Switchable Enantioselective Trapping of Carbamate Ammonium Ylides with Imines
Chiral Lithium(I) Binaphtholate Salts for the Enantioselective Direct Mannich-Type Reaction with a Change of Syn/Anti and Absolute Stereochemistry
Oxidative Mannich Reaction of N-Carbobenzyloxy Amines with 1,3-Dicarbonyl Compounds
Organocatalytic and Highly Stereoselective Direct Vinylogous Mannich Reaction
Pyridinium 1,1'-Binaphthyl-2,2'-disulfonates as Highly Effective Chiral Brønsted Acid-Base Combined Salt Catalysts for Enantioselective Mannich-Type Reaction
Asymmetric Mannich Reactions with in Situ Generation of Carbamate-Protected Imines by an Organic Catalyst
Cyclopropenimine-Catalyzed Enantioselective Mannich Reactions of tert-Butyl Glycinates with N-Boc-Imines
Direct-Type Catalytic Three-Component Mannich Reactions Leading to an Efficient Synthesis of α,β-Diamino Acid Derivatives
Asymmetric Organocatalytic Synthesis of 4-Aminoisochromanones via a Direct One-Pot Intramolecular Mannich Reaction






















No comments:
Post a Comment