Aldol Condensation
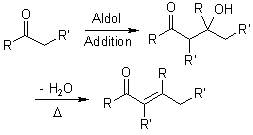
In some cases, the adducts obtained from the Aldol Addition can easily be converted (in situ) to α,β-unsaturated carbonyl compounds, either thermally or under acidic or basic catalysis. The formation of the conjugated system is the driving force for this spontaneous dehydration. Under a variety of protocols, the condensation product can be obtained directly without isolation of the aldol.
The aldol condensation is the second step of the Robinson Annulation.
Mechanism of the Aldol Condensation
For the addition step see Aldol Addition


Recent Literature





No comments:
Post a Comment