Aldol Reaction

'Aldol' is an abbreviation of aldehyde and alcohol. When the enolate of an aldehyde or a ketone reacts at the α-carbon with the carbonyl of another molecule under basic or acidic conditions to obtain β-hydroxy aldehyde or ketone, this reaction is called Aldol Reaction.
Mechanism of the Aldol Addition

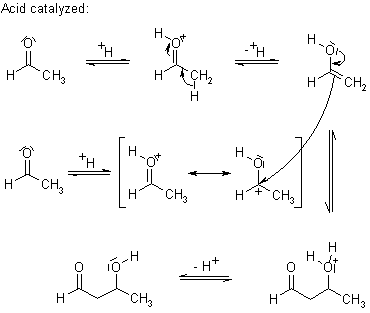
Under conditions of kinetic control, the mixed Aldol Addition can be used to prepare adducts that are otherwise difficult to obtain selectively. This process begins with the irreversible generation of the kinetic enolate, e.g. by employing a sterically hindered lithium amide base such as LDA (lithium diisopropylamide).
With an unsymmetrically substituted ketone, such a non-nucleophilic, sterically-demanding, strong base will abstract a proton from the least hindered side. Proton transfer is avoided with lithium enolates at low temperatures in ethereal solvents, so that addition of a second carbonyl partner (ketone or aldehyde) will produce the desired aldol product.

Recent Literature

Chiral Amine-Polyoxometalate Hybrids as Highly Efficient and Recoverable Asymmetric Enamine Catalysts

Enantioselective organocatalytic aldehyde–aldehyde cross-aldol couplings. The broad utility of α-thioacetal aldehydes

Combined Proline-Surfactant Organocatalyst for the Highly Diastereo- and Enantioselective Aqueous Direct Cross-Aldol Reaction of Aldehydes

Reconstructed Hydrotalcite as a Highly Active Heterogeneous Base Catalyst for Carbon-Carbon Bond Formations in the Presence of Water















No comments:
Post a Comment