What are Properties of Alcohol?
Alcohols are organic compounds in which a hydrogen atom of an aliphatic carbon is replaced with a hydroxyl group. Thus an alcohol molecule consists of two parts; one containing the alkyl group and the other containing functional group hydroxyl group. They have a sweet odour. They exhibit a unique set of physical and chemical properties.
The physical and chemical properties of alcohols are mainly due to the presence of hydroxyl group.
Physical Properties of Alcohol
1. The Boiling Point of Alcohols
Alcohols generally have higher boiling points in comparison to other hydrocarbons having equal molecular masses. This is due to the presence of intermolecular hydrogen bonding between hydroxyl groups of alcohol molecules. In general, the boiling point of alcohols increases with an increase in the number of carbon atoms in the aliphatic carbon chain. On the other hand, the boiling point decreases with an increase in branching in aliphatic carbon chains the Van der Waals forces decreases with a decrease in surface area. Thus primary alcohols have a higher boiling point.
2. Solubility of Alcohols
The solubility of alcohol in water is governed by the hydroxyl group present. The hydroxyl group in alcohol is involved in the formation of intermolecular hydrogen bonding. Thus, hydrogen bonds are formed between water and alcohol molecules which make alcohol soluble in water. However, the alkyl group attached to the hydroxyl group is hydrophobic in nature. Thus, the solubility of alcohol decreases with the increase in the size of the alkyl group.
3. The Acidity of Alcohols
Alcohols react with active metals such as sodium, potassium etc. to form the corresponding alkoxide. These reactions of alcohols indicate their acidic nature. The acidic nature of alcohol is due to the polarity of –OH bond. The acidity of alcohols decreases when an electron-donating group is attached to the hydroxyl group as it increases the electron density on the oxygen atom. Thus, primary alcohols are generally more acidic than secondary and tertiary alcohols. Due to the presence of unshared electrons on the oxygen atom, alcohols act as Bronsted bases too.
Chemical Properties of Alcohols
Alcohols exhibit a wide range of spontaneous chemical reactions due to the cleavage of the C-O bond and O-H bond. Some prominent chemical reactions of alcohols are:
1. Oxidation of Alcohol
- Alcohols undergo oxidation in the presence of an oxidizing agent to produce aldehydes and ketones which upon further oxidation give carboxylic acids.
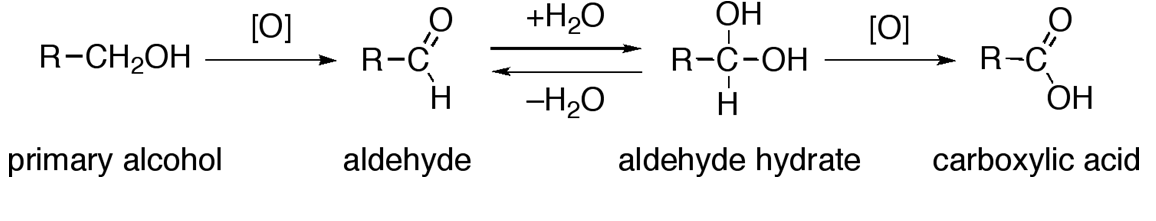
Alcohols: Physical and Chemical Properties
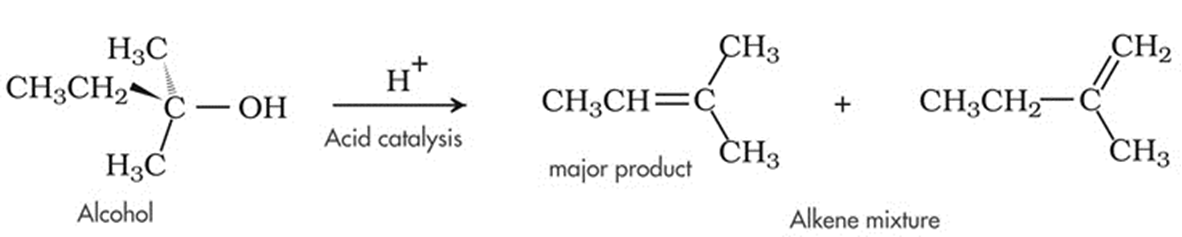
2. Dehydration of Alcohol
- Upon treatment with protic acids, alcohols undergo dehydration (removal of a molecule of water) to form alkenes. Dehydration of alcohol
No comments:
Post a Comment